When most people think about medicine, they imagine tiny pills, vials of vaccines, or packets of tablets sitting on pharmacy shelves. What many don’t realize is the intricate journey these products take before they reach the consumer — a journey that, for many medicines, must happen within a strictly controlled temperature range. This delicate process is known as pharma cold chain logistics, and it plays a vital role in ensuring that life-saving medications remain safe, effective, and ready for use when they’re needed most.
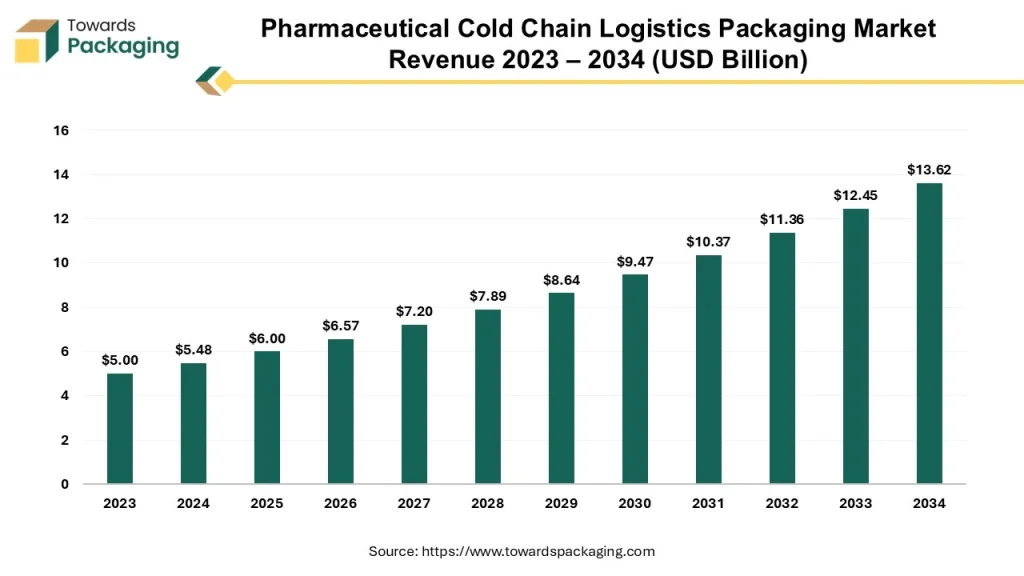
What Is Pharma Cold Chain Logistics?
Simply put, pharma cold chain logistics refers to the storage and transportation of temperature-sensitive pharmaceuticals under regulated conditions. Depending on the specific drug, temperature requirements can vary greatly:
-
Ultra-cold storage (-80°C to -60°C): For specialized products like certain mRNA vaccines.
-
Frozen storage (-25°C to -15°C): Common for some biologics.
-
Refrigerated storage (2°C to 8°C): Widely used for vaccines, insulin, and many injectables.
-
Controlled room temperature (15°C to 25°C): For certain oral medications and general pharmaceuticals.
Invest in Our Premium Strategic Solution: https://www.towardspackaging.com/download-databook/5330
If the temperature strays outside the recommended range during any stage — from manufacturing to transportation to storage — the product may become ineffective or even harmful.
Why Cold Chain Logistics Matter So Much
Protecting Patient Safety
The most obvious reason for strict cold chain control is patient safety. Medicines that degrade due to improper storage may lose their potency or develop harmful properties. For example, if a vaccine is not stored at the proper temperature, it may no longer provide immunity, putting countless lives at risk.
Meeting Global Regulations
Pharmaceutical companies must comply with strict guidelines from organizations like:
-
The U.S. FDA (Food and Drug Administration)
-
The European Medicines Agency (EMA)
-
The World Health Organization (WHO)
-
Good Distribution Practice (GDP) standards
These regulations are in place to ensure that all medications are transported and stored under conditions that preserve their quality and efficacy.
Get All the Details in Our Solutions – Access Report Preview: https://www.towardspackaging.com/download-sample/5330
Enabling Global Access
The pharmaceutical supply chain is global. A drug may be manufactured in one country, packaged in another, and distributed across continents. Cold chain logistics providers ensure these long, complex routes do not compromise the product.
Leading Companies in Pharma Cold Chain Logistics
Several specialized companies have built sophisticated systems to handle the unique challenges of pharmaceutical cold chain logistics. Here’s a look at some of the major players:
1. DHL Global Forwarding
DHL offers tailored pharmaceutical logistics under its Life Sciences & Healthcare division. Its Thermonet network connects multiple GDP-compliant facilities worldwide, equipped with cutting-edge temperature control systems and real-time monitoring to protect pharmaceutical shipments throughout their journey.
2. UPS Healthcare
UPS Healthcare has heavily invested in building a global cold chain infrastructure. With temperature-controlled warehouses, freezer farms, and high-tech monitoring tools, UPS ensures that even highly sensitive medications are transported and stored safely.
If you have any questions, please feel free to contact us at sales@towardspackaging.com
3. FedEx HealthCare Solutions
FedEx leverages its SenseAware® technology to offer complete visibility into shipment conditions like temperature, humidity, and shock during transit. Their extensive global network has made them a trusted partner in distributing critical pharmaceutical supplies.
4. Kuehne+Nagel
Through its KN PharmaChain service, Kuehne+Nagel provides comprehensive GDP-compliant logistics solutions for the healthcare sector. They employ IoT-enabled monitoring for real-time data on temperature and location to guarantee safety throughout the cold chain.
5. Marken
A specialized subsidiary of UPS, Marken focuses on clinical trial logistics, cell and gene therapy distribution, and direct-to-patient delivery services. Their global network supports highly specialized, sensitive cold chain solutions for cutting-edge therapies.
6. Cryoport
Cryoport specializes in ultra-cold chain logistics for advanced cell and gene therapies. With expertise in cryogenic temperatures (as low as -150°C), they play a key role in delivering complex biological therapies safely and efficiently.
The Technology Behind Pharma Cold Chain
Managing temperature-sensitive pharmaceuticals requires precision and constant monitoring. Thanks to technology, today’s cold chain logistics are more reliable than ever:
-
IoT Sensors: These devices track temperature, location, humidity, and more in real-time, alerting logistics teams if any issues arise.
-
Data Analytics: Predictive analytics help optimize routes, improve efficiency, and prevent delays or temperature excursions.
-
Blockchain: Provides secure, transparent tracking of every stage of a shipment’s journey.
-
Advanced Packaging: Innovations in insulated packaging and phase-change materials help maintain stable temperatures even in challenging conditions.
Challenges Facing Pharma Cold Chain Logistics
While technology has greatly advanced the industry, cold chain logistics still faces several challenges:
-
Limited Infrastructure in Developing Regions: Many parts of the world lack reliable cold chain facilities, making global distribution more difficult.
-
High Costs: Maintaining ultra-cold storage and monitoring systems is expensive, especially for long-distance or rural deliveries.
-
Regulatory Complexity: Different countries have varying regulations, requiring expert navigation of compliance requirements.
-
Sustainability Concerns: Traditional cold chain systems are energy-intensive, prompting companies to invest in greener, more efficient solutions.
The Pandemic’s Impact: Cold Chain Under the Spotlight
The COVID-19 pandemic brought unprecedented attention to the pharmaceutical cold chain industry. Vaccines had to be delivered globally, often requiring ultra-cold storage conditions never previously attempted at such scale. Cold chain companies rapidly scaled up infrastructure, integrated new technologies, and coordinated with governments to ensure efficient vaccine rollout — all while navigating a global crisis.
The lessons learned during the pandemic have strengthened the industry, leading to long-term improvements in cold chain capacity, technology adoption, and global collaboration.
The Future of Pharma Cold Chain Logistics
As pharmaceutical research advances, cold chain logistics will continue to evolve:
-
Personalized Medicine: More customized treatments will require highly specialized handling.
-
AI and Automation: Predictive AI tools will enhance risk management and improve operational efficiency.
-
Sustainable Solutions: Eco-friendly refrigeration, reusable packaging, and green energy sources will become more common.
-
Global Equity: Continued investment will help bring life-saving drugs to underserved regions around the world.
Conclusion
Pharma cold chain logistics companies are the silent heroes of global healthcare. Their work ensures that life-saving medications — from routine vaccines to cutting-edge gene therapies — arrive safely, wherever and whenever they are needed.
In a world increasingly reliant on complex, temperature-sensitive pharmaceuticals, the importance of robust, reliable cold chain logistics cannot be overstated. As innovation drives healthcare forward, cold chain logistics will remain at the heart of delivering better outcomes for patients worldwide.

